Description
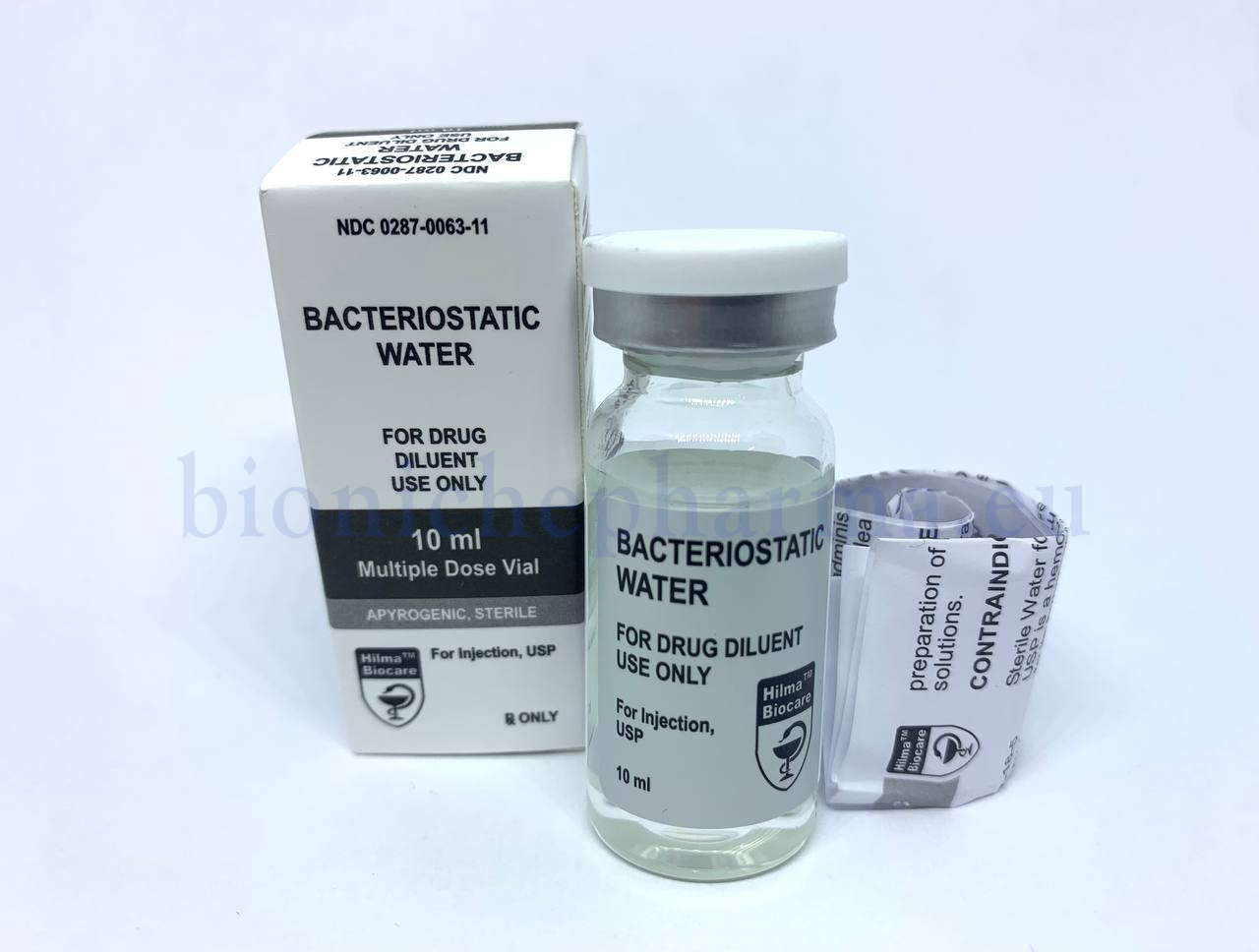
Bacteriostatic Water
ATC code: V07AB
Bacteriostatic Water
ATC code: V07AB
CAS number:7732-18-5
Dosage Form: Injectable
Market Status: Prescription
Company: Hilma Biocare
CLINICAL PHARMACOLOGY
Sterile Water for Injection, USP is used for fluid replacement only after suitable additives are
introduced to approximate isotonicity and to serve as a vehicle for suitable medications.
INDICATIONS AND USAGE
Sterile Water for Injection, USP is indicated in the aseptic preparation of parenteral solutions.
CONTRAINDICATIONS
Sterile Water for Injection, USP is a hemolytic agent due to its hypotonicity. Therefore, it is
contraindicated for intravenous administration without additives.
WARNINGS
Do not use intravenous injection unless adjusted to approximate isotonicity with a suitable
solute. Hemolysis may occur following infusion of Sterile Water for Injection, USP.
Hemoglobin induced renal failure has been reported following hemolysis.
PRECAUTIONS
Do not administer unless the solution is clear and the seal is intact.
ADVERSE REACTIONS
The administration of a suitable admixture of prescribed additives may be associated
reactions because of the solution or the technique of administration including febrile
response, infection at the site with adverse of injection, venous thrombosis or phlebitis
extending from the site of injection, extravasation, and hypervolemia. If an adverse reaction
does occur, discontinue the infusion. evaluate the patient, institute appropriate therapeutic
countermeasures, and save the remainder of the fluid for examination if deemed necessary.
DOSAGE AND ADMINISTRATION
Following suitable admixture of prescribed additives, the dosage is usually dependent upon
the age, weight and clinical condition of the patient as well as laboratory determinations. See
directions accompanying additive drugs. Do not store an unused portion of Sterile Water for
Injection, USP. Mix thoroughly when additives have been introduced. Do not store solutions
containing additives.
PRESENTATION
Sterile water, 10ml multiple dose vial.
STORAGE
Store between 20 – 25°C.
7732-18-5
Dosage Form: Injectable
Market Status: Prescription
Company: Hilma Biocare
CLINICAL PHARMACOLOGY
Sterile Water for Injection, USP is used for fluid replacement only after suitable additives are
introduced to approximate isotonicity and to serve as a vehicle for suitable medications.
INDICATIONS AND USAGE
Sterile Water for Injection, USP is indicated in the aseptic preparation of parenteral solutions.
CONTRAINDICATIONS
Sterile Water for Injection, USP is a hemolytic agent due to its hypotonicity. Therefore, it is
contraindicated for intravenous administration without additives.
WARNINGS
Do not use intravenous injection unless adjusted to approximate isotonicity with a suitable
solute. Hemolysis may occur following infusion of Sterile Water for Injection, USP.
Hemoglobin induced renal failure has been reported following hemolysis.
PRECAUTIONS
Do not administer unless the solution is clear and the seal is intact.
ADVERSE REACTIONS
The administration of a suitable admixture of prescribed additives may be associated
reactions because of the solution or the technique of administration including febrile
response, infection at the site with adverse of injection, venous thrombosis or phlebitis
extending from the site of injection, extravasation, and hypervolemia. If an adverse reaction
does occur, discontinue the infusion. evaluate the patient, institute appropriate therapeutic
countermeasures, and save the remainder of the fluid for examination if deemed necessary.
DOSAGE AND ADMINISTRATION
Following suitable admixture of prescribed additives, the dosage is usually dependent upon
the age, weight and clinical condition of the patient as well as laboratory determinations. See
directions accompanying additive drugs. Do not store an unused portion of Sterile Water for
Injection, USP. Mix thoroughly when additives have been introduced. Do not store solutions
containing additives.
PRESENTATION
Sterile water, 10ml multiple dose vial.
STORAGE
Store between 20 – 25°C.


Reviews
There are no reviews yet.